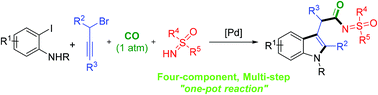
One-pot multi-step cascade protocols toward β-indolyl sulfoximidoyl amides via intermolecular trapping of an α-indolylpalladium complex by CO†
Abstract
Various β-indolyl sulfoximidoyl amides were efficiently prepared from ortho-iodoanilines, propargyl bromides, 1 atm of CO, and substituted NH-sulfoximines, through a palladium-catalyzed indole annulation/carbonyl insertion/C–N bond formation cascade. Mostly good to high yields of the products were obtained through this multi-step, one-pot reaction protocol under very gentle reaction conditions. The obtained β-indolyl sulfoximidoyl amides could be converted into biologically interesting sulfoximine analogues that contain a tryptamine moiety.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...