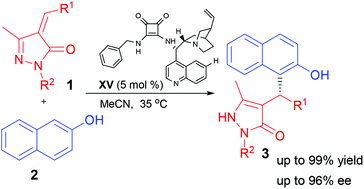
Abstract
The first method for highly efficient asymmetric Michael-type Friedel–Crafts alkylation of naphthol and unsaturated pyrazolones has been accomplished under mild reaction conditions. In the presence of the chiral squaramide catalyst, a wide range of substrates are tolerated in excellent yields (up to 99%) with reasonable enantioselectivities (up to 96% ee).
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...