Mixed NHC–thiolato complexes of palladium: understanding the formation of di-versus mononuclear complexes†
Abstract
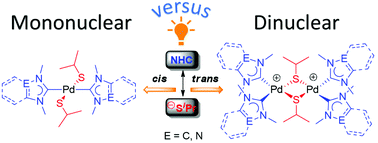
The preference for the formation of mono- versus dinuclear mixed carbene/thiolato complexes of PdII has been studied with three types of N-heterocyclic carbenes derived from benzimidazole, imidazole and 1,2,4-triazole. The complexes were prepared by treatment of halido/NHC precursors with sodium isopropylthiolate in a salt metathesis reaction. Mononuclear complexes are formed when the sulfur and carbon donors are exclusively cis to each other, while their trans arrangement preferably leads to dinuclear complexes with μ2-bridging thiolato ligands. The increased electron density in the latter case cannot be sufficiently compensated by one PdII center alone and leads to the formation dinuclear species with bridging thiolato ligands.


 Please wait while we load your content...
Please wait while we load your content...