Atomic layer deposition of thin-film sodium manganese oxide cathode materials for sodium ion batteries
Abstract
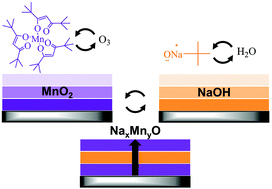
To improve the performance of sodium ion batteries (NIBs), we need to better understand the materials chemistry occurring at the surface of NIB cathode materials. In this work, we aim to form thin films of sodium manganese oxide (NMO) cathode materials for NIBs using atomic layer deposition (ALD) with the vision to isolate and study these interfacial processes in the absence of bulk NMO. We combine established chemistries for ALD of manganese oxide (MnOx) using Mn(thd)3/O3 and sodium hydroxide (NaOH) using NaOtBu/H2O and adjust the sequence and ratios of these two chemistries to form NaxMnyO alloy films. We identify that increasing the O3 exposure during Mn(thd)3/O3 ALD beyond previously reported values increases the growth rate of MnOx from 0.23 to 0.62 Å per cycle and provides improved uniformity, yielding predominantly Mn5O8. Furthermore, alloying Mn(thd)3/O3 with NaOtBu/H2O mutually enhances the growth rate of both ALD chemistries, yielding a growth rate of ∼9 Å per supercycle for a 1 : 1 cycle ratio. This enhancement in growth arises from sub-surface reactions, including the reaction of NaOtBu to a depth of ∼1.3 nm into bulk MnOx to form Na2MnOx. By tuning cycle ratios and growth conditions, we demonstrate control over the NaxMnyO composition and measure different electrochemical properties depending on the composition. The formation of NMO thin films with controlled thickness and composition established in this work provides a means to systematically study interfacial processes occurring in NMO cathode materials for NIBs.
- This article is part of the themed collection: Spotlight Collection: Atomic and Molecular Layer Deposition


 Please wait while we load your content...
Please wait while we load your content...