The cation and anion bonding modes make a difference: an unprecedented layered structure and a tri(hetero)nuclear moiety in thioantimonates(v)†
Abstract
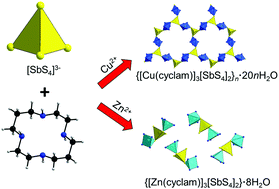
Mixing solutions of M2+ (M = Cu2+ or Zn2+) salts containing cyclam (cyclam = 1,4,8,11-tetraazacyclotetradecane) as the ligand and an aqueous solution of Na3SbS4·9H2O at room temperature led to the crystallization of two new compounds within minutes: {[Cu(cyclam)]3[SbS4]2}n·20nH2O (I) and {[Zn(cyclam)]3[SbS4]2}·8H2O (II). In the structure of I [SbS4]3− anions acting as a tridentate ligand join CuN4S2 octahedra generating twelve-membered rings by corner-sharing of SbS4 and CuN4S2 units. The rings are condensed into layers, which are stacked onto each other in a 6R polytype manner. The layers contain large pores with the water molecules located between the layers above and below the pores. In contrast, the structure of II comprises a discrete molecular tri(hetero)nuclear moiety with a bidentate [SbS4]3− anion connecting two rectangular pyramidal ZnN4S polyhedra. The crystal water molecules of I and II can be thermally removed, and I and II are recovered by treatment under a humid atmosphere. The EPR spectrum of I indicates the presence of Cu2+ cations, which is unusual in the environment of S2− anions. The different bonding situations and the preferences for the coordination geometries of Cu2+ and Zn2+ cations are rationalized by DFT based calculations, demonstrating that Cu2+ prefers an octahedral environment while Zn2+ adopts the square-pyramidal coordination. The pronounced differences in the vibrational spectra are also analyzed with DFT, showing how the different modes are influenced by the differing bond strengths.


 Please wait while we load your content...
Please wait while we load your content...