Practical access to fluorescent 2,3-naphthalimide derivatives via didehydro-Diels–Alder reaction†
Abstract
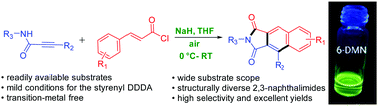
A practical and efficient approach for the synthesis of fluorescent 2,3-naphthalimide derivatives has been developed from readily available starting materials via an intramolecular didehydro-Diels–Alder reaction, which proceeded well under room temperature, exhibiting a wide substrate scope and good functional group tolerance. The practicability of this methodology has been verified by one-step synthesis of the environmentally sensitive fluorophore 6-DMN on a gram scale with a shorter time, fewer steps and less waste disposal, and without the utilization of toxic transition metals. The present experimental and computational studies support the crucial role of the propiolimide moiety in the transformation.


 Please wait while we load your content...
Please wait while we load your content...