The manner and extent to which the hydration shell impacts interactions between hydrated species
Abstract
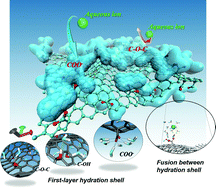
The hydration shell (HS) has a critical impact on every contact between hydrated species, which is a prerequisite for a great many physical and chemical processes, such as ion adsorption at the solution–solid interface. This paper reveals the extent and manner to which the HS interferes with ion adsorption utilizing molecular dynamics. The single-layer HS is the smallest unit that maintains the ionic hydration structure and the force on it. The energy penalty incurred by partial dehydration upon adsorption is one of the approaches through which HS influences ion adsorption, yet the collision of water molecules in HS may be the critical one. The repulsive force during dehydration is, to great extent, neutralized by HS collision. The index for estimating the extent of the influence of the HS is not the hydration energy, but the quantification of the contest between HS’ collision and the binding of adsorption sites. The hydration energy is larger for charged functional groups, but the HS’ impact is much smaller, as compared with electroneutral group cases. As a result, the order of the adsorption capacity for different ionic species may be quite different between charged and electroneutral cases.

 Please wait while we load your content...
Please wait while we load your content...