NMR study on the cellulose dissolution mechanism in CaCl2·6H2O–LiCl molten salt hydrate†
Abstract
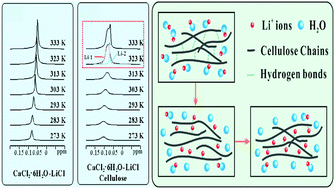
As there is a rising interest in upgrading cellulose to high-performance bio-products, the studies on innovative reaction media and processes have been leaping forward. Green solvents in terms of cellulose dissolution and brief processes for upgrading are critical to green chemistry. However, most solvent systems generally exhibit defects in harsh pH operating windows with limited temperature ranges, environmental pollution, long reaction times, complicated processes, etc. In this work, we have provided a novel molten salt hydrate (CaCl2·6H2O–LiCl) as a green solvent and investigated the role of hydrated molten salts in the dissolution process via the solid state nuclear magnetic resonance (NMR) technique. The cellulose could be dissolved in CaCl2·6H2O–LiCl molten salt hydrated at 120 °C with 3.0% solubility and regenerated in-situ by cooling down to ambient temperature. The regenerated cellulose exhibited a high solubility and excellent stability. From 7Li single pulse NMR experiments, it was observed that two types of Li+ existed in the cellulose dissolution, and the Li+ significantly impacted the dissolving process and the dissolution ability of cellulose. This work would provide an environmental-friendly strategy to prepare cellulose solutions for biocompatible cellulose materials.


 Please wait while we load your content...
Please wait while we load your content...