The critical role of configurational flexibility in facilitating reversible reactive metal deposition from borohydride solutions†
Abstract
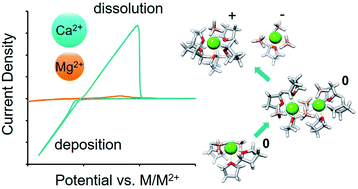
Development of calcium metal batteries has been historically frustrated by a lack of electrolytes capable of supporting reversible calcium electrodeposition. In this paper, we report the study of an electrolyte consisting of Ca(BH4)2 in tetrahydrofuran (THF) to gain important insight into the role of the liquid solvation environment in facilitating the reversible electrodeposition of this highly reactive, divalent metal. Through interrogation of the Ca2+ solvation environment and comparison with Mg2+ analogs, we show that an ability to reversibly electrodeposit metal at reasonable rates is strongly regulated by dication charge density and polarizability. Our results indicate that the greater polarizability of Ca2+ over Mg2+ confers greater configurational flexibility, enabling ionic cluster formation via neutral multimer intermediates. Increased concentration of the proposed electroactive species, CaBH4+, enables rapid and stable delivery of Ca2+ to the electrode interface. This work helps set the stage for future progress in the development of electrolytes for calcium and other divalent metal batteries.


 Please wait while we load your content...
Please wait while we load your content...