Ru-doped, oxygen-vacancy-containing CeO2 nanorods toward N2 electroreduction †
Abstract
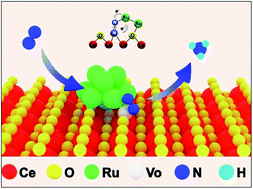
Electrochemical N2 reduction (N2RR) in aqueous solutions under ambient conditions is extremely challenging; thus, a rational design of electrocatalytic centers is required to effectively adsorb and activate N2. Herein, we report Ru cluster-doped, oxygen vacancy-rich CeO2 (Ru/CeO2-VO) nanorods as an efficient N2 reduction electrocatalyst. The high-density oxygen vacancies inside CeO2 are beneficial for the adsorption of N2, and the unsaturated, low-valence Ru nanoclusters provide active sites for N2 activation and subsequent electron transfer for N2 reduction. The Ru/CeO2-VO catalyst exhibits a high ammonia production rate of 9.87 × 10−8 mmol s−1 cm−2 and a partial current density of 32 μA cm−2 at −0.25 V versus reversible hydrogen electrode in 0.05 M H2SO4 solution, corresponding to a faradaic efficiency of 11.7%. Our work suggests that the synergistic effects between Ru nanoclusters and oxygen vacancies in CeO2 for efficient N2 fixation.


 Please wait while we load your content...
Please wait while we load your content...