Metal free decarboxylative aminoxylation of carboxylic acids using a biphasic solvent system†
Abstract
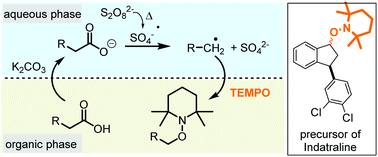
The smooth oxidative radical decarboxylation of carboxylic acids with TEMPO and other derivatives as radical scavengers is reported. The key to success was the use of a two-phase solvent system to avoid otherwise predominant side reactions such as the oxidation of TEMPO by persulfate and enabled the selective formation of synthetically useful alkoxyamines. The method does not require transition metals and was successfully used in a new synthetic approach for the antidepressant indatraline.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...