Synthesis of spirocyclic heterocycles from α,β-unsaturated N-acyliminium ions†
Abstract
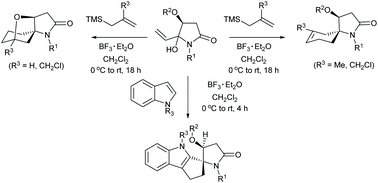
The reactions of α,β-unsaturated N-acyliminium ions, generated in situ from 4(S)-O-substitutedhydroxy-5-hydroxy-5-vinyl-N-alkylpyrrolidin-2-ones, with allylsilanes and indoles leading to the formation of spirocyclic heterocycles, are reported. Six single crystal X-ray structures and extensive 2D NMR experiments confirmed the structures and stereochemistries of these products. In addition, computational studies provided mechanistic insights and an understanding of the stereochemical outcomes of these reactions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...