Chiral cyclometalated iridium complexes for asymmetric reduction reactions†
Abstract
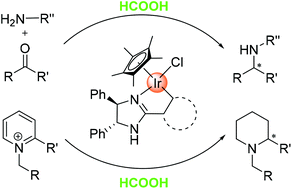
A series of chiral cyclometalated iridium complexes have been synthesised by cyclometalating chiral 2-aryl-oxazoline and imidazoline ligands with [Cp*IrCl2]2. These iridacycles were studied for asymmetric transfer hydrogenation reactions with formic acid as the hydrogen source and were found to display various activities and enantioselectivities, with the most effective ones affording up to 63% ee in the asymmetric reductive amination of ketones and 77% ee in the reduction of pyridinium ions.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...