Heat- or light-induced acylarylation of unactivated alkenes towards 3-(α-acyl) indolines†
Abstract
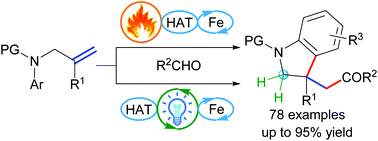
A heat- or photoredox/iron dual catalysis-enabled dehydrogenative acylarylation of N-allyl anilines leading to 2-substituted 3-(α-acyl) indolines with a quaternary stereogenic center is presented, with unactivated alkenic bonds as radical acceptors and simple aldehydes as radical precursors. This reaction features high yields, a broad substrate scope, and a great exo selectivity, and gram-scale syntheses could be readily carried out.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...