Sulfur-mediated annulation of 1,2-phenylenediamines towards benzofuro- and benzothieno-quinoxalines†
Abstract
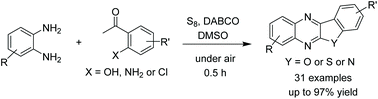
We report a method for condensation between ortho-phenylenediamines and ortho-hydroxyacetophenones to afford benzofuroquinoxalines. The reactions proceeded in the presence of an elemental sulfur mediator, DABCO base, and DMSO solvent. Functionalities such as nitrile, ester, and halogen groups were compatible. The conditions could be applicable for the synthesis of benzothienoquinoxalines from ortho-chloroacetophenones.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...