Preparation of 4-arylquinazolines with o-(N-alkyl,N-p-tosyl)aminobenzonitriles, aryllithiums, and NIS†
Abstract
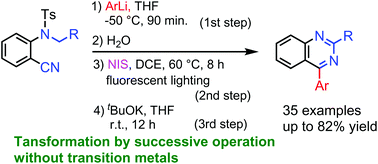
The treatment of o-(N-alkyl,N-p-tosyl)aminobenzonitriles with aryllithiums, followed by the reaction with water, NIS under irradiation with a fluorescent lamp, and then tBuOK gave 2-alkyl-4-arylquinazolines or 4-arylquinazolines in good to moderate yields. The present reaction proceeds through the formation of N-iodoimines from imines with NIS, the generation of iminyl radicals, the 1,6-H shift by iminyl radicals, the cyclization via 6-exo-tet mode, and finally the elimination of p-toluenesulfinate to generate 2-alkyl-4-arylquinazolines or 4-arylquinazolines.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...