Condensation of 1,2-dicarbonyl compounds with modified Huisgen zwitterions: synthesis of N-aryl–N-acyl hydrazones†
Abstract
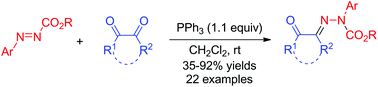
A phosphine-mediated deoxygenative condensation of 1,2-dicarbonyl compounds such as aroylformates, α-diketones, and isatins with arylazocarboxylates has been developed for a facile synthesis of N-aryl–N-acyl hydrazones in moderate to excellent yields under very mild conditions. Mechanistic investigation based on 31P NMR tracking experiments unveils that the reaction is initiated with the in situ formation of the modified Huisgen zwitterions from arylazocarboxylates and PPh3 and proceeds via a nitrogen to nitrogen ester group migration process. This study also represents the first exploration of the reactivity patterns of the modified Huisgen zwitterions derived from arylazocarboxylates toward electrophiles such as 1,2-dicarbonyl compounds.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...