Selective synthesis of trisubstituted pyrroles through the reactions of alkynyl Fischer carbene complexes with oxazolones†
Abstract
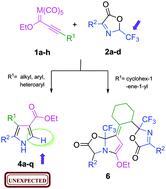
An efficient and simple synthesis of novel trisubstituted 1H-pyrroles 4a–qvia 1,3-dipolar cycloaddition of Δ3-trifluoromethyloxazolones 2a–d with both chromium and tungsten alkynyl Fischer carbene complexes (1a–h) is described. An unexpected and unreported –CF3 group elimination process was observed in the pyrrole structure. Our experimental and theoretical data suggested that the metal fragment may be responsible for this phenomenon. The dipolar cycloaddition proceeded efficiently to produce a single regioisomer, which was unambiguously established through NMR and single-crystal X-ray diffraction studies. Nevertheless, the reaction of alkynyl carbenes bearing an α,β,γ,δ-unsaturated moiety with excess oxazolone 2a produced a polycyclic compound 6 speculatively formed through a cascade reaction involving 1,6-, 1,4- and 1,2-nucleophilic addition steps.


 Please wait while we load your content...
Please wait while we load your content...