Pt-Ligand single-atom catalysts: tuning activity by oxide support defect density†
Abstract
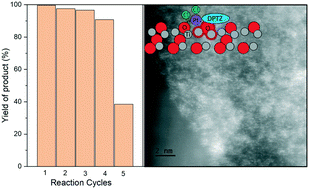
The support–metal interaction is an important element of heterogeneous catalyst design and is particularly critical for the rapidly growing field of single-atom catalysts (SACs). We investigate the impact of varying the defect density of the titania support on metal–organic Pt SACs for hydrosilylation reactions. Pt SACs are decorated on the powder titania support, employing a metal–ligand interaction with a dipyridyl-tetrazine ligand (DPTZ). The single-atom nature of Pt is verified by X-ray absorption spectroscopy (XAS) on both pristine and defective titania surfaces. These Pt species have a +2 oxidation state and are stabilized by bonding with DPTZ, surface oxygen, and residual chloride from the metal precursor. The catalytic activity is evaluated for the alkene hydrosilylation reaction and it is discovered that the activity of Pt-ligand is positively correlated with the defect density of the titania support. The turn-over number (TON) is calculated to be 12 530 for Pt SACs on a defective surface, which is significantly higher than Pt SACs on a pristine titania surface (830) for the same reaction period (10 min) under the same conditions (70 °C). We ascribe the high activity of Pt SACs on defective titania surfaces to two aspects: the coordination of Pt with more chloride than on pristine titania surfaces, which shortens the induction period of the reaction, and the preferential dispersion of Pt–DPTZ units on defective regions of titania surfaces, allowing facile contact between Pt sites and reactants. The supported Pt–DPTZ SACs show high stability through multiple cycles of batch reactions. This work demonstrates an efficient approach to improve the activity and stability of SACs by optimizing the metal–support interaction, which can also be applied to other oxide surfaces to further develop next-generation heterogeneous single-atom catalysts.


 Please wait while we load your content...
Please wait while we load your content...