Origin of CO2 as the main carbon source in syngas-to-methanol process over Cu: theoretical evidence from a combined DFT and microkinetic modeling study†
Abstract
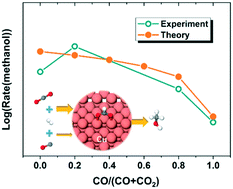
The main carbon source for methanol synthesis from syngas (CO, CO2 and H2) has been studied experimentally for decades. In the current work, we attempt to understand the origin of such observations at a molecular level by combining density functional theory (DFT) and a microkinetic modeling approach. The energetics of elementary steps in the process of CO and CO2 hydrogenation to methanol on Cu(211) were calculated by DFT. We found that the hydrogenation of CO to methanol over Cu(211) proceeds via the reaction pathway of CO → HCO → CH2O → CH3O → CH3OH. Subsequently, microkinetic modeling was performed, taking a mixture of CO, CO2 and H2 as the reactant, with a variable ratio between CO and CO2. The microkinetic results showed that the Cu(211) surface will have a certain coverage (around 0.083 monolayer) of spectating HCOO at the steady state. More importantly, the formation rate of methanol will increase dramatically with a small amount of CO2 added to the mixture of CO and H2 over the surface with spectating HCOO, which is in good agreement with previous experimental results. In addition, we found that the formation of methanol from CO2 hydrogenation is always faster than that from CO hydrogenation, which further supports the conclusion that the main carbon source in the syngas-to-methanol process over Cu catalyst is CO2, rather than CO.


 Please wait while we load your content...
Please wait while we load your content...