Two-way desorption coupling to enhance the conversion of syngas into aromatics by MnO/H-ZSM-5†
Abstract
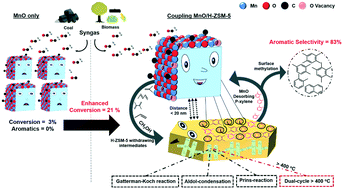
Aromatics are some of the most important bulk chemicals used in modern industries, and rapid economic development and environmental issues that originate from the use of fossil fuels force us to search for clean and sustainable ways to produce fuels and chemicals from high carbon and renewable resources like syngas. We herein report a composite catalyst containing partially reducible and highly active manganese oxide and nano-size H-ZSM-5 with short b-axis, prepared for the direct conversion of syngas into aromatics. We achieved high aromatic selectivity of 83% at a stable CO conversion of 21% for at least 200 hours without any deactivation. Manganese oxide not only helped in C–O bond activation and formation of methanol, alkenes and dienes, but it also helped in aromatic desorption from H-ZSM-5 pores. On the other hand, H-ZSM-5 facilitated the immediate removal of intermediates from MnOx into its pores and converted them into aromatics. This two-way desorption capability of the composite catalyst enhanced conversion and selectivity when the components were present at a distance of a few nm away from each other. The key intermediate species during the induction time were found to be cyclopentenones, furans, aldehydes and OH containing compounds. These intermediate species were converted into aromatics through multiple reaction routes, such as the Gattermann–Koch reaction, aldol-condensation and the Prins reaction. The factors responsible for the consistent performance and long life of the composite catalyst were the nano-size H-ZSM-5 with short b-axis, the aromatic desorption ability of manganese oxide, and the presence of H2 in the reaction system. This work paves the way for the use of single metal oxide catalysts for efficient aromatization of syngas in a single step process.


 Please wait while we load your content...
Please wait while we load your content...