Hydroboration of internal alkynes catalyzed by FeH(CO)(NO)(PPh3)2: a case of boron-source controlled regioselectivity†
Abstract
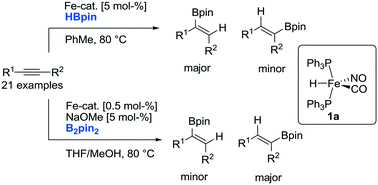
The structurally defined Fe–H complex FeH(CO)(NO)(Ph3P)2 catalyzes the efficient stereoselective hydroboration of a variety of internal alkynes using either pinacolborane (HBpin) or bis(pinacolato)diboron (B2pin2) as a boron source. Upon hydroboration of unsymmetrical internal alkynes, a boron-source dependent regioselectivity was observed. Whereas the use of HBpin provides access to products that can be rationalized through a borylferration mechanism, a complementary regioselectivity was observed using B2pin2, which is indicative of a hydroferration mechanism.


 Please wait while we load your content...
Please wait while we load your content...