Oxidation of biomass-derived furans to maleic acid over nitrogen-doped carbon catalysts under acid-free conditions†
Abstract
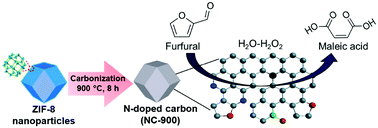
Maleic acid (MA) is a platform chemical used for synthesizing high-value polymers. Although MA can be produced from biomass-derived furfural, so far, only acidic catalysts have been proposed. Herein, for the first time, we report an acid-free furfural-to-MA conversion using nitrogen-doped carbon (NC) as an effective catalyst with the assistance of hydrogen peroxide (H2O2) as an oxidant. A high MA yield (61%) can be achieved over the NC-900 catalyst at 80 °C. The effects of reaction conditions, including H2O2 concentration, solvent type, reaction temperature, and time were systematically studied in detail and optimized. The ZIF-8-derived NC catalyst exhibited high activity owing to the existence of high graphitic nitrogen (N-Q) content in the carbon framework. The kinetics study indicates that the oxidation of furfural to MA over the NC-900 catalyst is via the formation of 5-hydroxy-furan-2(5H)-one as the main intermediate. Further, NC-900 can convert a variety of biomass-derived furan compounds to MA under acid-free conditions, indicating the versatility and applicability of the present acid-free, NC-based catalytic system.


 Please wait while we load your content...
Please wait while we load your content...