Selective catalytic oxidation of ammonia over LaMAl11O19−δ (M = Fe, Cu, Co, and Mn) hexaaluminates catalysts at high temperatures in the Claus process†
Abstract
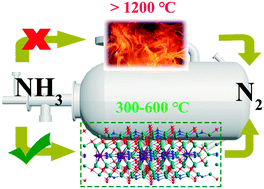
A method for the selective catalytic oxidation of ammonia at high temperature was innovatively proposed to substitute the traditional combustion method to remove the ammonia impurity in the Claus process. In the present work, transition metal (Fe, Cu Co, and Mn)-substituted La-hexaaluminate catalysts were synthesized and investigated for the selective catalytic oxidation of ammonia (NH3-SCO) at high temperature. It was observed that Cu-substituted catalysts could achieve the highest N2 yield at around 520 °C. It was confirmed that the conversion of NH3 was closely related to the reducibility of the prepared catalyst. In particular, it was observed that the molecular O2 could not be dissociatively adsorbed on the prepared catalyst surface. However, both lattice oxygen and gas-phase O2 could participate in NH3-SCO, with gas-phase O2 being the most favorable under the experimental conditions. It was evidenced that the NH3-SCO reaction over the prepared catalysts followed the i-SCR mechanism. Moreover, monodentate nitrates were the main reactive intermediates toward forming N2. Therefore, the development of high-temperature SCO technology and an efficient catalyst are beneficial for the sustainable development of the chemical industry.


 Please wait while we load your content...
Please wait while we load your content...