A low-cost anodic catalyst of transition metal oxides for lithium extraction from seawater†
Abstract
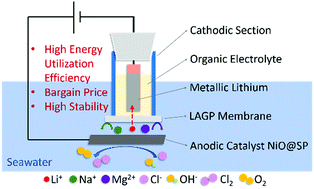
Lithium reserves in seawater are tens of thousands of times higher than on land, making it a promising candidate for lithium resources. A lithium extraction method based on a solar-powered electrolysis technique with a solid-state electrolyte, Li1.5Al0.5Ge1.5(PO4)3 (LAGP), as the selective membrane has been reported to obtain metallic lithium from seawater. Herein, the electrolytic cell is optimised by replacing the anode catalyst materials. The NiO@SP anode shows excellent electrochemical performance, relatively high energy utilization efficiency and low cost among the anode materials investigated. An electrolytic cell adopting NiO@SP achieves a lithium production efficiency of 57.2 mg W h−1 with a potential of 4.5 V at a current density of 333 μA cm−2. Based on the investigation by in situ mass spectroscopy the oxygen evolution reaction (OER) and chlorine evolution reaction (CER) occur together on the anode with the production of oxygen and hypochlorite.


 Please wait while we load your content...
Please wait while we load your content...