Bi(iii)-catalyzed aminooxygenation of propargyl amidines to synthesize 2-fluoroalkyl imidazole-5-carbaldehydes and their decarbonylations†
Abstract
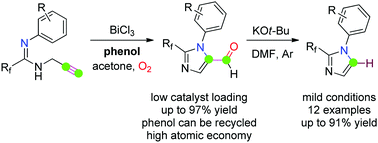
The first example of main group metal Bi(III)-catalyzed aminooxygenation of fluorinated propargyl amidines was developed to produce 2-fluoroalkyl imidazole-5-carbaldehydes in moderate to excellent yields, in which phenol played a critical role and could be recovered and recycled. In the presence of KOt-Bu, an unconventional decarbonylation occurred on the 2-fluoroalkyl imidazole-5-carbaldehydes.


 Please wait while we load your content...
Please wait while we load your content...