Synthesis of aryl-substituted 2-methoxyphenol derivatives from maltol-derived oxidopyrylium cycloadducts through an acid-mediated ring contraction cascade†
Abstract
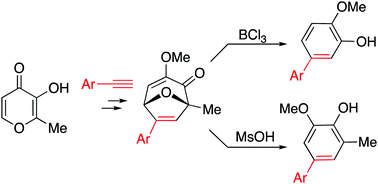
Oxidopyrylium cycloadducts derived from maltol and aryl acetylenes undergo acid-mediated rearrangements to generate aryl-substituted 2-methoxyphenol (guaiacol) derivatives. Specifically, the cycloadducts react with boron trichloride to form 2-methoxy-5-arylphenol molecules, and with methane sulfonate to form 2-methoxy-4-aryl-6-methylphenol molecules.


 Please wait while we load your content...
Please wait while we load your content...