Indium phosphasalen catalysts showing high isoselectivity and activity in racemic lactide and lactone ring opening polymerizations†
Abstract
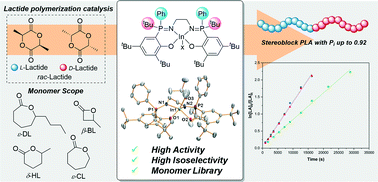
Stereoblock polylactide (PLA) shows higher melting temperatures and better mechanical properties than other PLA stereoisomers. More stereoselective and active catalysts are needed to polymerize racemic-lactide (LA) and produce stereoblock PLA. This work describes a series of phosphasalen indium catalysts (1–5) which result in very high isoselectivity, at room temperature, (Pi = 0.91, 25 °C) and high activity, at low catalyst loading (TOF = 100 h−1, 1 : 500 catalyst : LA, [LA] = 1 M, THF, 25 °C). The catalyst structure–activity and structure–stereoselectivity relationships are investigated using various experimental methods and DFT calculations. The most isoselective catalyst features two different phosphasalen substituents, a tert-butyl and phenyl group, it forms an achiral, meso indium complex which operates by a chain end control mechanism. The work highlights the benefits of phosphasalen ligands and identifies new avenues for catalyst investigation by exploitation of asymmetrically substituted phosphorus atoms. The catalysts also show good activity and control for the ring-opening polymerizations of ε-caprolactone, β-butyrolactone, ε-decalactone and δ-hexalactone (γ-methyl-δ-valerolactone), demonstrating future potential for copolyester production.


 Please wait while we load your content...
Please wait while we load your content...