The role of surface properties in CO2 methanation over carbon-supported Ni catalysts and their promotion by Fe†
Abstract
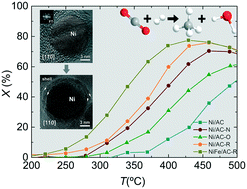
The surface chemistry of the activated carbon supporting material was tailored to investigate the influence of O and N groups as well as O-free Lewis basic sites on the performance of Ni-based catalysts for CO2 methanation. Catalytic experiments demonstrated that 15% Ni on carbon with O-free Lewis basic sites showed the best performance (XCO2 = 76%, SCH4 = 97%, T = 450 °C, P = 1 bar), suggesting that the methanation reaction is highly dependent on the basicity of the support. Promotion with 5% Fe enabled the decrease of the optimal reaction temperature while improving the stability of the catalyst. Microstructural and compositional studies of the catalyst after a prolonged time on stream experiment suggested that the slight but gradual catalyst deactivation during methanation is associated with the sintering of Ni nanoparticles and the development of a distinct Ni@NiO core–shell nanostructure.


 Please wait while we load your content...
Please wait while we load your content...