Chemodivergent reaction of azomethine imines and 2H-azirines for the synthesis of nitrogen-containing scaffolds†
Abstract
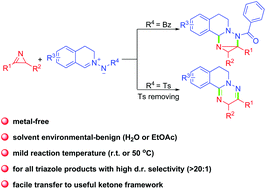
The metal-free reactions of 2H-azirines with C,N-cyclic azomethine imines were investigated. N-Bridged strained ring-fused triazole derivatives or 1,2,4-triazine derivatives are obtained, and the chemoselectivity is dependent on the protecting group on the azomethine dipole. Although benzoyl-protected starting materials give access to strained polycyclic compounds, a ring-opening occurs in the case of a tosyl protecting group that is cleaved during the process with elimination of sulfinic acid. 1,2,4-Triazine can be prepared on a mmol-scale and concomitantly oxidized with air as an oxidant to form the corresponding ketone, which is an interesting structural motif that can be found in bioactive scaffolds.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...