Copper nitrate-mediated synthesis of 3-aryl isoxazolines and isoxazoles from olefinic azlactones†
Abstract
A copper nitrate-mediated [2 + 2 + 1] cycloaddition reaction was developed for the expedient synthesis of pharmacologically interesting 3-aryl substituted isoxazolines and isoxazoles through C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage. Copper nitrate is employed as a reaction promoter and precursor of nitrile oxides. The given approach features a new mode of cycloaddition from olefinic azlactones, copper nitrate and unsaturated compounds with wide substrate scope, good functional group tolerance and operational simplicity.
C bond cleavage. Copper nitrate is employed as a reaction promoter and precursor of nitrile oxides. The given approach features a new mode of cycloaddition from olefinic azlactones, copper nitrate and unsaturated compounds with wide substrate scope, good functional group tolerance and operational simplicity.
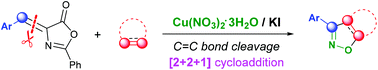
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...