Planarized B,N-phenylated dibenzoazaborine with a carbazole substructure: electronic impact of the structural constraint†
Abstract
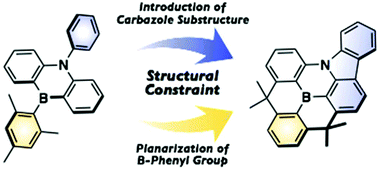
A B,N-diphenyl-5,10-dihydro-dibenzo-1,4-azaborine, in which both phenyl groups on the boron and nitrogen atoms are planarized to generate a carbazole substructure, was synthesized. The structral constraint around the boron and nitrogen atoms alters the π-conjugation mode and thus the photophysical and electrochemical properties. Specifically, this structurally constrained dibenzoazaborine showed an intense blue emission with a narrow full width at half maximum. One of its derivatives exhibited near infrared absorption in the one-electron-oxidized state.
- This article is part of the themed collection: Trends in Organoboron Chemistry


 Please wait while we load your content...
Please wait while we load your content...