Catalyst-free regioselective hydroxyfluorination and aminofluorination of α,β-unsaturated ketones†
Abstract
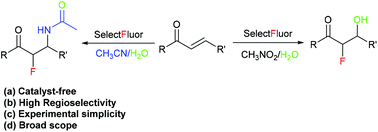
The catalyst-free direct regioselective α-fluoro-β-hydroxylation and α-fluoro-β-amidation of α,β-unsaturated ketones has been developed. Various α,β-unsaturated ketones react with Selectfluor in water and acetonitrile to give α-fluorohydrins and α-fluoroamides respectively with moderate to good yields. The mechanistic studies revealed the possibility of a radical based pathway.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...