Chiral benzazaboroles as catalysts for enantioselective sulfonylation of cis-1,2-diols†
Abstract
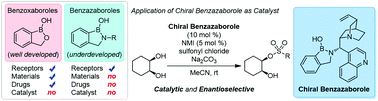
A newly developed benzazaborole smoothly catalyzed the enantioselective sulfonylation of cis-1,2-diols. Using a chiral benzazaborole/NMI co-catalyst system, various sulfonate esters were prepared in high yields with good enantioselectivities.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...