Electrochemical trifluoromethylation/semipinacol rearrangement sequences of alkenyl alcohols: synthesis of β-CF3-substituted ketones†
Abstract
Electrochemical oxidative radical trifluoromethylation/semipinacol rearrangement sequences of alkenyl alcohols were developed in this study. This approach is environmentally benign and uses the shelf-stable Langlois reagent as a trifluoromethyl radical precursor and electrons as the oxidizing reagents. The present protocol offers a facile route to prepare β-trifluoromethylated ketone derivatives.
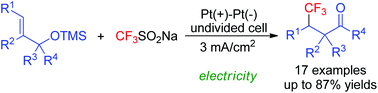
- This article is part of the themed collections: Synthetic methodology in OBC and Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...