PdII-Catalyzed methoxylation of C(sp3)–H bonds adjacent to benzoxazoles and benzothiazoles†
Abstract
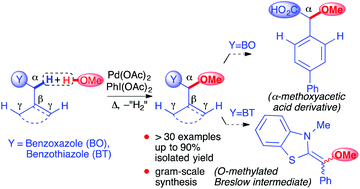
The Pd(OAc)2/PhI(OAc)2 catalyst system promotes the highly regioselective dehydrogenative methoxylation of a C(sp3)–H bond adjacent to benzoxazole and benzothiazole rings. The title transformation constitutes the first example of a Pd-catalyzed C(sp3)–H activating methoxylation at the proximal-selective α-position with regard to a directing auxiliary and provides expedient access to an important class of azole-decorated methyl ethers (up to 90% isolated yield). The synthetic practicality of the methodology was demonstrated by achieving α-methoxyacetic acids via the elimination of the benzoxazole auxiliaries and by obtaining the precursor of an O-methylated Breslow intermediate.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...