Physicochemical interactions with (−)-epigallocatechin-3-gallate drive structural modification of celiac-associated peptide α2-gliadin (57–89) at physiological conditions†
Abstract
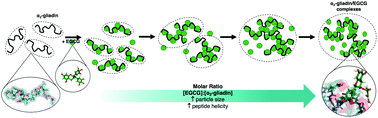
(−)-Epigallocatechin-3-gallate (EGCG), a major phenolic constituent of tea, has been shown to have biological activity within inflammatory pathways involved with food allergies and intolerances. Proposed mechanisms for this effect include sequestration and structural modification of immunostimulatory proteins as a result of interactions with EGCG. The present study employs biophysical techniques including dynamic light scattering, circular dichroism and nuclear magnetic resonance to elucidate the likely mechanism(s) by which EGCG interacts with α2-gliadin (57–89) (α2g), an immunodominant peptide in celiac disease pathogenesis. We demonstrate that EGCG interacts with α2g in a multi-phase reaction driven by non-specific binding, resulting in the formation of polydisperse EGCG/α2g complexes which induce changes in peptide structure. We also show that these interactions occur at a range of pH levels associated with digestion, including pH 2.0, 6.8 and 7.5. Based on previous reports of binding specificity of enzymes and antigen presenting cells in celiac disease pathogenesis, our results provide foundational support for EGCG to prevent recognition of immunostimulatory gliadin epitopes by the body and thus prevent the inflammatory and autoimmune response associated with celiac disease.


 Please wait while we load your content...
Please wait while we load your content...