Predicting aromatic exciplex fluorescence emission energies†
Abstract
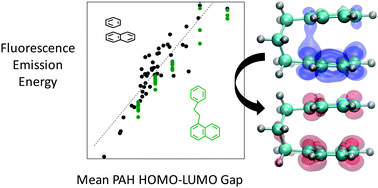
PAH dimerization has been widely posited to play an important, even rate-determining role in soot nucleation, despite scanty experimental evidence of the existence of PAH dimers in flames. Laser-induced fluorescence (LIF) offers a promising in situ method of identifying PAH dimers, if dimer fluorescence can be distinguished from the fluorescence of the constituent monomers and other species present. Predicting transition energies for excited dimers (excimers) and excited complexes (exciplexes) represents a significant challenge for theory. Nonempirically tuned LC-BLYP functionals have been used to compute excited-state geometries and emission energies for a database of 81 inter- and intramolecular PAH excimers and exciplexes. Exciplex emission energies depend sensitively on the topology of the PAHs involved, but a linear relationship between the mean monomer bandgap and the computed exciplex emission means that dimer electronic properties can be predicted based on the properties of the constituent monomers. The range of fluorescence energies calculated for structures containing small to moderately-sized PAHs indicates that either noncovalent or aliphatically-linked complexes could generate the visible-range fluorescence energies observed in LIF experiments.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...