Detailed kinetic model for hexyl sulfide pyrolysis and its desulfurization by supercritical water†
Abstract
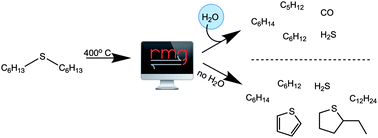
A detailed reaction network is proposed for the pyrolysis and desulfurization of hexyl sulfide in the presence or absence of both supercritical water (SCW) and hexadecane, but without any added H2 or catalyst, for T = 400–450 °C. The new kinetic model is developed using the Reaction Mechanism Generator (RMG) software where most of the rate coefficients are derived from quantum chemical calculations. We previously reported that pentane, carbon monoxide and carbon dioxide are major products of hexyl sulfide desulfurization in SCW, but not in the anhydrous pyrolysis of hexyl sulfide. The observation of CO and CO2 in the reaction products indicates that water effectively acts as a hydrogen source; presumably this assists in sulfur reduction to H2S. Kinetic parameters for several of the important reactions are calculated using transition state theory and quantum chemical calculations at the CBS-QB3 level of theory and then further refined using CCSD(T)-F12//cc-pVTZ-F12 single point energies. Predictions from the new kinetic model agree with factor-of-2 accuracy with new and previously published experimental data for hexyl sulfide conversion and for yields of most major products, either neat or in a hexadecane solvent, both in the presence and absence of SCW. Flux analysis was then used to identify the most important reaction steps, and sensitivity analysis was used to propose reactions that should be studied further in the future to decrease the model's uncertainty. This study establishes the molecular role of water as diluent, hydrogen bond donor, and reductant in the decomposition of hexyl sulfide. Future work to add molecular weight growth pathways to the model would lead to a more complete mechanism, resulting in improved predictions of product yields.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...