Influence of monomer deformation on the competition between two types of σ-holes in tetrel bonds†
Abstract
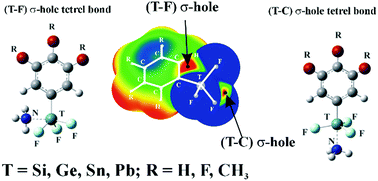
One of several tetrel (T) atoms was covalently attached to three F atoms and a substituted phenyl ring. A NH3 base can form a tetrel bond with TF3C6H2R3 (T = Si, Ge, Sn, Pb; R = H, F, CH3) in a position opposite either an F atom or the ring. The σ-hole opposite the highly electron-withdrawing F (T–F) is more intense than that opposite the ring (T–C). However, when the Lewis base deforms from a tetrahedral to a trigonal bipyramidal shape so as to accommodate the base, it is the T–C σ-hole that is more intense. Accordingly, it is the T–C tetrel-bonded complex for which there is a larger interaction energy with NH3, as high as 34 kcal mol−1. Countering this trend, it requires more energy for the TF3C6H2R3 to deform into the geometry it adopts within the T–C complex than within its T–F counterpart. There is consequently a balance between the overall binding energies of the two competing sites. The smaller tetrel atoms Si and Ge, with their larger deformation energies, show a preference for T–F tetrel binding, while the T–C site is preferred by Pb which suffers from a smaller degree of deformation energy. There is a near balance for T = Sn and the two sites show comparable binding energies.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...