Triplet state promoted reaction of SO2 with H2O by competition between proton coupled electron transfer (pcet) and hydrogen atom transfer (hat) processes†
Abstract
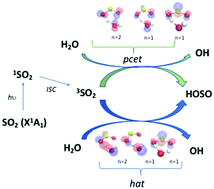
The SO2 + H2O reaction is proposed to be the starting process for the oxidation of sulfur dioxide to sulfate in liquid water, although the thermal reaction displays a high activation barrier. Recent studies have suggested that the reaction can be promoted by light absorption in the near UV. We report ab initio calculations showing that the SO2 excited triplet state is unstable in water, as it immediately reacts with H2O through a water-assisted proton coupled electron transfer mechanism forming OH and HOSO radicals. The work provides new insights for a general class of excited-state promoted reactions of related YXY compounds with water, where Y is a chalcogen atom and X is either an atom or a functional group, which opens up interesting chemical perspectives in technological applications of photoinduced H-transfer.


 Please wait while we load your content...
Please wait while we load your content...