Shock wave and modelling study of the dissociation pathways of (C2F5)3N†
Abstract
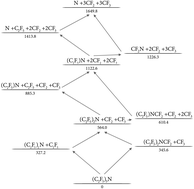
The thermal decomposition of perfluorotriethylamine, (C2F5)3N, was investigated in shock waves by monitoring the formation of CF2. Experiments were performed over the temperature range of 1120–1450 K with reactant concentrations between 100 and 1000 ppm of (C2F5)3N in the bath gas Ar and with [Ar] in the range of (0.7–5.5) × 10−5 mol cm−3. The experiments were accompanied by quantum-chemical calculations of the energies of various dissociation paths and by rate calculations, in particular for the dissociation of C2F5via C2F5 → CF3 + CF2. The overall reaction can proceed in different ways, either by a sequence of successive C–N bond ruptures followed by fast C2F5 decompositions, or by a sequence of alternating C–C and C–N bond ruptures. A cross-over between the two pathways can also take place. At temperatures below about 1300 K, yields of less than one CF2 per (C2F5)3N decomposed were observed. On the other hand, at temperatures around 2000 K, when besides the parent molecule, CF3 also dissociates, yields of six CF2 per (C2F5)3N decomposed were measured. The rate-delaying steps of the dissociation mechanism at intermediate temperatures were suggested to be the processes (C2F5)NCF2 → (C2F5)N + CF2 and (CF2)N → N + CF2. The reduction of the CF2 yields at low temperatures was tentatively attributed to a branching of the mechanism at the level of (C2F5)2NCF2, from where the cyclic final product perfluoro-N-methylpyrrolidine, (C4F8)NCF3, is formed which was identified in earlier work from the literature.


 Please wait while we load your content...
Please wait while we load your content...