Unraveling ultrafast dynamics of the photoswitchable bridged dithienylethenes under structural constraints†
Abstract
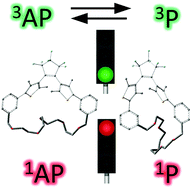
The excited state dynamics of constrained photochromic benzodithienylethenes were addressed by considering the bridging with polyether chains (from x = 4 to 6 units) at the ortho and meta positions of the aryl group, named DTE-ox and DTE-mx, via time-resolved absorption spectroscopy supported with (TD)-DFT calculations. The photochromic parameters and geometrical structures of these series are discussed. A novel photocyclization pathway via a triplet state, evidenced recently (Hamdi et al., Phys. Chem. Chem. Phys., 2016, 18, 28091–28100), is largely dependent on the length and the position of the polyether chain. For the first time, by comparing the two series, we revealed, for the DTE-ox series, an interconversion not only in the ground state but also between the triplet states of the anti-parallel and parallel open form conformers.


 Please wait while we load your content...
Please wait while we load your content...