On the estimation of crystallization driving forces†
Abstract
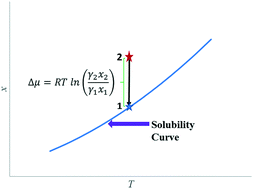
For accurate estimation of crystallization driving forces, activity coefficient ratios need to be estimated. Activity coefficients in saturated solutions can be determined from solubility data if accurate pure component thermodynamic data are available. However, activity coefficients in supersaturated solutions can normally not be determined experimentally. In this paper, we investigate whether standard activity coefficient models fitted to data on saturated solutions can be used for estimation of activity coefficients in supersaturated solutions. For this purpose, we use equilibrium data for 9 polymorphic systems, for which activity coefficients can be extracted from the solubility data for each polymorph, respectively. The 3-parameter NRTL and the 2-parameter Wilson equations are fitted to the data for the more stable form, and are then used to predict the activity coefficients in the solution saturated by the metastable form, i.e. a solution that is supersaturated with respect to the stable form. It is shown that the predictions of the activity coefficients in the saturated solution of the metastable form deviate significantly from the corresponding experimental data. The activity coefficients along the solubility curve of one polymorphic form do not contain sufficient information to distinguish the influence of concentration from the influence of temperature, and accordingly the fitted equation cannot provide an independent characterisation of the influence of concentration on the activity coefficient. If the data for two polymorphic forms are used simultaneously in the regression, the models can provide a better estimation of the true driving force in crystallization processes.


 Please wait while we load your content...
Please wait while we load your content...