Synthesis, coordination chemistry and photophysical properties of naphtho-fused pyrazole ligands†
Abstract
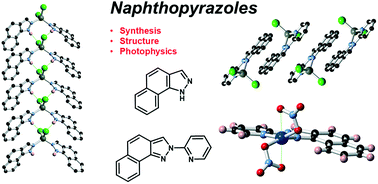
The synthesis of two π-extended pyrazole ligands is reported, with naphtho[2,1-d]-1H-pyrazole HL1 prepared in a modified Jacobson indazole synthesis from 1-amino-2-methylnaphthalene, and subsequent arylation with 2-bromopyridine giving the chelating ligand N-(2-pyridyl)-naphtho[1,2-c]pyrazole L2 as a single isomer, with both species crystallographically characterised. Each forms mononuclear complexes with Cu2+ and Zn2+; in [Cu(HL1)4(NO3)2] 1 and [ZnCl2(HL1)2] 2 the propensity for outer-sphere hydrogen bonding from the pyrazole N–H group supplements the extended π backbone in influencing crystal packing interactions. The equivalent complexes of the chelating L2, [Cu(L2)(NO3)2] 3 and [ZnCl2(L2)] 4, both show distortion at the coordination sphere caused by the close approach of the hydrogen atom at the naphthyl 8-position, and crystal packing in both instances is dictated purely by the flat aromatic backbone of the ligand. Stability constants for complexes 3 and 4 are determined spectroscopically, and photophysical studies reveal fluorescence with vibrational progressions in both the solution and solid state for each ligand and the zinc complexes 2 and 4.
- This article is part of the themed collection: Introducing the CrystEngComm Advisory Board and their research


 Please wait while we load your content...
Please wait while we load your content...