Formation and stability of well-crystallized metastable octacalcium phosphate at high temperature by regulating the reaction environment with carbamide†
Abstract
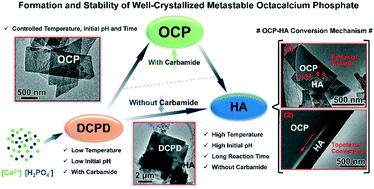
Octacalcium phosphate (OCP), an important intermediate precursor of biological apatite, is an ideal model to help understand the biomineralisation mechanism. However, it is difficult to obtain pure OCP and evaluate its characteristics because of its metastable phase. In this work, pure well-crystallized OCP was successfully prepared by a precipitation method using carbamide as a precipitator. The carbamide concentration and reaction temperature synergistically affected the holistic reaction kinetics, whereas the initial pH and reaction time regulated the extent of formation and stability of the OCP crystals. At a higher temperature, high-dose carbamide hydrolyzed to produce more OH− to accelerate the early formation of the intermediate phase (brushite), phase transitions, and crystallization. In this respect, higher initial pH facilitated the rapid transition from brushite to OCP, which gradually converted to hydroxyapatite through the coexisting mechanisms of epitaxial growth and topotaxial conversion. Particularly, unhydrolyzed carbamide could also stabilize newly formed pure OCP crystals even at 90 °C. This work paves the way for understanding the biomineralisation process in vivo and the development of biomaterials for bone regeneration using OCP.


 Please wait while we load your content...
Please wait while we load your content...