Liquid–liquid extraction in flow of the radioisotope titanium-45 for positron emission tomography applications†
Abstract
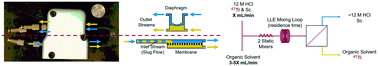
A continuous liquid–liquid extraction of natTi and its PET radioisotope 45Ti into an organic phase from 12 M HCl is described. The extraction is completely selective with respect to Sc, which is commonly used as a cyclotron target for 45Ti production. A membrane-based separator with integrated pressure control allowed for efficient, reproducible, and robust aqueous/organic phase separation in flow. Optimization studies established a guaiacol–anisole 9/1 (v/v) mixture and a flow rate ratio of 1/3 (aq. to org.), with a residence time of 13.7 s as the optimal extraction conditions. 90.3 ± 1.1% of natTi was consistently extracted from a 0.01 M solution of natTiCl4 and ScCl3, while 84.8 ± 2.4% of 45Ti was extracted from 0.03–0.13 M ScCl3 containing picomolar amounts of the 45Ti radionuclide, without extracting any Sc from either system. The organic phase can be directly used for 45Ti-radiolabelling as demonstrated by the efficient radiosynthesis of the 45Ti-radiolabeled antineoplastic [45Ti](salan)Ti(dipic). This development opens a pathway to achieve continuous and efficient 45Ti recovery and processing using an automated micro or millifluidics setup.


 Please wait while we load your content...
Please wait while we load your content...