Kinetic analysis of the steam reforming of ethanol over Ni/SiO2 for the elucidation of metal-dominated reaction pathways
Abstract
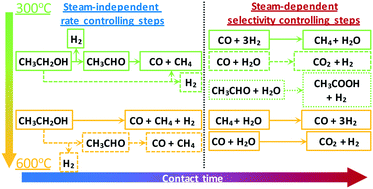
Hydrogen production via steam reforming of biomass-derived ethanol is a promising environmental alternative to the use of fossil fuels and a means of clean power generation. A kinetic study of ethanol steam reforming (ESR) is presented, where the effect of temperature, space time and partial pressure of reactants is investigated over a wide range in a fixed-bed reactor over a Ni/SiO2 catalyst. The order of the reaction was found to be 0.5 in ethanol and almost zero in water, indicating a steam-independent rate-limiting step, while an apparent activation energy of 48 kJ mol−1 was obtained. Identification of primary and secondary products revealed the reaction mechanism to be strongly affected by temperature with results suggesting the existence of two alternate pathways being active, one involving acetaldehyde and one an ethanol decomposition derived surface intermediate. Below 450 °C, ethanol decomposition and dehydrogenation were found to be dominant, whereas at higher temperatures secondary methane steam reforming (MSR) and water-gas shift (WGS) reactions became enhanced. An excess of water was able to promote the WGS reaction and suppress the methanation reaction even at 400 °C. Time-on-stream studies at 500 °C revealed Ni/SiO2 to have a good balance between stability, activity and selectivity in ESR. Temperature-programmed oxidation (TPO) and hydrogenation (TPH) analyses indicated that the carbonaceous deposits were graphitic in nature, suggesting the presence of filamentous coke.


 Please wait while we load your content...
Please wait while we load your content...