One-pot synthesis of 2-naphthols from nitrones and MBH adducts via decarboxylative N–O bond cleavage†
Abstract
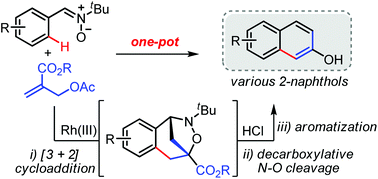
The efficient synthesis of 2-naphthols is important for their further development as bioactive compounds and chiral ligands as well as other synthetic purposes. Herein, we describe the unprecedented one-pot synthesis of 2-naphthols through an acid-mediated decarboxylative N–O bond cleavage of bridged benzoxazepine intermediates, which were in turn generated from aryl nitrones and Morita–Baylis–Hillman (MBH) adducts under cationic rhodium(III) catalysis. A range of 2-naphthol derivatives including anthracen-2-ol, phenanthren-2-ol, and 11H-benzo[b]fluoren-7-ol were formed with excellent site selectivities and functional group compatibilities. To gain mechanistic insight into this process, a series of mechanistic investigations and DFT calculations were also performed.


 Please wait while we load your content...
Please wait while we load your content...