Mild dealkylative N-nitrosation of N,N-dialkylaniline derivatives for convenient preparation of photo-triggered and photo-calibrated NO donors†
Abstract
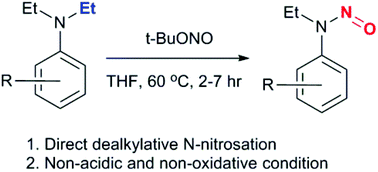
N-Nitroso push–pull dyes are unique NO donors, whose NO release is triggered by light and accompanied by a concomitant fluorescence turn-on. Their synthesis involves nitrosation of the corresponding secondary amines, which, however, are not readily available. In this manuscript, we developed a mild, convenient and high-yielding preparation of N-nitroso aryl amines via dealkylative N-nitrosation of tertiary amines. This method is readily employed for the preparation of N-nitrosated rhodamine and coumarin dyes, whose potential as novel NO donors were showcased in cultured cell lines.


 Please wait while we load your content...
Please wait while we load your content...